Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Irisawa, Eriko; Kato, Chiaki
Journal of Nuclear Materials, 591, p.154914_1 - 154914_10, 2024/04
Times Cited Count:0The amount of corrosion of austenitic stainless-steel R-SUS304ULC was evaluated considering the changes in solution composition and boiling during actual concentration operations. Austenitic stainless-steel R-SUS304ULC is the structural material of the highly radioactive liquid waste concentrator in Japanese spent fuel reprocessing plant, which treats highly corrosive nitric acid solutions during enrichment operations. The study results show that it is necessary to focus on nitric acid concentrations, oxidizing metal ion concentrations, and decompression boiling as factors that accelerate the corrosion rate of stainless steel because of cathodic reaction activation.
Suzuki, Tomoya*; Otsubo, Ukyo*; Ogata, Takeshi*; Shiwaku, Hideaki; Kobayashi, Toru; Yaita, Tsuyoshi; Matsuoka, Mitsuaki*; Murayama, Norihiro*; Narita, Hirokazu*
Separation and Purification Technology, 308, p.122943_1 - 122943_7, 2023/03
Times Cited Count:2 Percentile:24.43(Engineering, Chemical)HNO leaching is used in recycling Pd metal from spent products that primarily contain Ag, and most Pd residues are separated from solutions containing Ag(I). However, a small amount of Pd(II) often remains in these Ag(I) solutions. Therefore, the separation of Pd(II) and Ag(I) in HNO
leaching is used in recycling Pd metal from spent products that primarily contain Ag, and most Pd residues are separated from solutions containing Ag(I). However, a small amount of Pd(II) often remains in these Ag(I) solutions. Therefore, the separation of Pd(II) and Ag(I) in HNO solutions is essential to promote efficient Pd recycling. In this study, the separation of Pd(II) and Ag(I) in HNO
solutions is essential to promote efficient Pd recycling. In this study, the separation of Pd(II) and Ag(I) in HNO solutions was investigated using four N-donor-type adsorbents functionalized with amine (R-Amine), iminodiacetic acid (R-IDA), pyridine (R-Py), or bis-picolylamine (R-BPA). R-Amine, R-IDA, and R-Py selectively adsorbed Pd(II) over Ag(I), Cu(II), Ni(II), and Fe(III) from HNO
solutions was investigated using four N-donor-type adsorbents functionalized with amine (R-Amine), iminodiacetic acid (R-IDA), pyridine (R-Py), or bis-picolylamine (R-BPA). R-Amine, R-IDA, and R-Py selectively adsorbed Pd(II) over Ag(I), Cu(II), Ni(II), and Fe(III) from HNO solutions (0.3-7 M), but R-Amine exhibited a lower Pd adsorption efficiency. In contrast,
solutions (0.3-7 M), but R-Amine exhibited a lower Pd adsorption efficiency. In contrast,  90% of Pd(II), Ag(I), and Cu(II) were adsorbed by R-BPA over the entire range of HNO
90% of Pd(II), Ag(I), and Cu(II) were adsorbed by R-BPA over the entire range of HNO concentrations. Structural analyses of the adsorbed metal ions using Fourier transform infrared spectroscopy and extended X-ray absorption fine structure spectroscopy revealed the separation mechanisms of the N-donor-type adsorbents. Pd(II) adsorption on R-IDA, R-Py, and R-BPA occurred via Pd(II) coordination of the functional groups (iminodiacetic acid, pyridine, and bis-picolylamine, respectively), whereas that on R-Amine occurred via anion exchange of NO
concentrations. Structural analyses of the adsorbed metal ions using Fourier transform infrared spectroscopy and extended X-ray absorption fine structure spectroscopy revealed the separation mechanisms of the N-donor-type adsorbents. Pd(II) adsorption on R-IDA, R-Py, and R-BPA occurred via Pd(II) coordination of the functional groups (iminodiacetic acid, pyridine, and bis-picolylamine, respectively), whereas that on R-Amine occurred via anion exchange of NO
 with [Pd(NO
with [Pd(NO )
) ]
] . The coordinative adsorption mechanisms resulted in the higher Pd(II) adsorption behaviors of R-IDA, R-Py, and R-BPA. HCl (5.0 M) and thiourea (0.1 M) eluents desorbed 83% of Pd(II) from R-IDA and 95% from R-Py, respectively. R-Py was the most effective Pd(II) adsorbent based on adsorption selectivity and desorption efficiency.
. The coordinative adsorption mechanisms resulted in the higher Pd(II) adsorption behaviors of R-IDA, R-Py, and R-BPA. HCl (5.0 M) and thiourea (0.1 M) eluents desorbed 83% of Pd(II) from R-IDA and 95% from R-Py, respectively. R-Py was the most effective Pd(II) adsorbent based on adsorption selectivity and desorption efficiency.
Matsuda, Shohei; Yokoyama, Keiichi; Yaita, Tsuyoshi; Kobayashi, Toru; Kaneta, Yui; Simonnet, M.; Sekiguchi, Tetsuhiro; Honda, Mitsunori; Shimojo, Kojiro; Doi, Reisuke; et al.
Science Advances (Internet), 8(20), p.eabn1991_1 - eabn1991_11, 2022/05
Times Cited Count:6 Percentile:58.16(Multidisciplinary Sciences)no abstracts in English
 Np
NpIrisawa, Eriko; Kato, Chiaki; Yamashita, Naoki; Sano, Naruto
Zairyo To Kankyo, 71(3), p.70 - 74, 2022/03
In order to evaluate the corrosion of stainless steels used in spent nuclear fuel reprocessing facilities, the immersion corrosion tests and polarization measurements were performed using R-SUS304ULC stainless steel in nitric acid solution containing a kind of radionuclides,  Np. At temperatures above 328 K, the corrosion potential was higher than that in nitric acid solution and was near the transpassive region. From the comparison between the corrosion amount calculated by the immersion corrosion tests and the polarization resistance, the values of
Np. At temperatures above 328 K, the corrosion potential was higher than that in nitric acid solution and was near the transpassive region. From the comparison between the corrosion amount calculated by the immersion corrosion tests and the polarization resistance, the values of 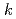 =0.018-0.025 V were obtained as a conversion factor, and the possibility of calculating the corrosion amount from the electrochemical measurement was examined.
=0.018-0.025 V were obtained as a conversion factor, and the possibility of calculating the corrosion amount from the electrochemical measurement was examined.
Irisawa, Eriko; Yamamoto, Masahiro; Kato, Chiaki; Motooka, Takafumi; Ban, Yasutoshi
Journal of Nuclear Science and Technology, 56(4), p.337 - 344, 2019/04
Times Cited Count:6 Percentile:48.99(Nuclear Science & Technology)Takahatake, Yoko; Ambai, Hiromu; Sano, Yuichi; Takeuchi, Masayuki; Koizumi, Kenji; Sakamoto, Kan*; Yamashita, Shinichiro
Proceedings of Annual Topical Meeting on Reactor Fuel Performance (TopFuel 2018) (Internet), 9 Pages, 2018/10
The corrosion behaviour of FeCrAl-ODS steels for the accident tolerant fuel cladding of LWRs were investigated in nitric acid solutions for the reprocessing process of spent fuels. The corrosion tests were carried out at 60 C, 80
C, 80 C and the boiling point of the solutions, and the specimens were then analysed by XPS. The corrosion remarkably progressed at the boiling point, and the highest corrosion rate was 0.22 mm/y. In the oxide film, the atomic concentration of Fe was lower, than that in the base material, and those of Cr and Al were higher. The results show that the corrosion of FeCrAl-ODS steels in hot nitric acid solution is not severe because of the high corrosion resistance of the oxide film formed on the material; hence, the corrosion resistance of the new cladding materials in the dissolution process of spent fuel is acceptable for reprocessing operations.
C and the boiling point of the solutions, and the specimens were then analysed by XPS. The corrosion remarkably progressed at the boiling point, and the highest corrosion rate was 0.22 mm/y. In the oxide film, the atomic concentration of Fe was lower, than that in the base material, and those of Cr and Al were higher. The results show that the corrosion of FeCrAl-ODS steels in hot nitric acid solution is not severe because of the high corrosion resistance of the oxide film formed on the material; hence, the corrosion resistance of the new cladding materials in the dissolution process of spent fuel is acceptable for reprocessing operations.
 -ray irradiation
-ray irradiationSano, Yuichi; Ambai, Hiromu; Takeuchi, Masayuki; Iijima, Shizuka; Uchida, Naoki
Journal of Nuclear Materials, 493, p.200 - 206, 2017/09
Times Cited Count:7 Percentile:56.89(Materials Science, Multidisciplinary)Concerning the Fukushima Daiichi Nuclear Power Plant accident, we investigated the effect of chloride ion on the corrosion behavior of SUS316L stainless steel, which is a typical material for the equipment used in reprocessing, in HNO solution containing seawater components, including under the
solution containing seawater components, including under the  -ray irradiation condition. Electrochemical and immersion tests were carried out using a mixture of HNO
-ray irradiation condition. Electrochemical and immersion tests were carried out using a mixture of HNO and artificial seawater (ASW). In the HNO
and artificial seawater (ASW). In the HNO solution containing high amounts of ASW, the cathodic current densities increased and uniform corrosion progressed. This might be caused by strong oxidants, such as Cl
solution containing high amounts of ASW, the cathodic current densities increased and uniform corrosion progressed. This might be caused by strong oxidants, such as Cl and NOCl, generated in the reaction between HNO
and NOCl, generated in the reaction between HNO and Cl
and Cl ions. The corrosion rate decreased with the immersion time at low concentrations of HNO
ions. The corrosion rate decreased with the immersion time at low concentrations of HNO , while it increased at high concentrations. Under the
, while it increased at high concentrations. Under the  -ray irradiation condition, the corrosion rate decreased due to the suppression of the cathodic reactions by the reaction between the above oxidants and HNO
-ray irradiation condition, the corrosion rate decreased due to the suppression of the cathodic reactions by the reaction between the above oxidants and HNO generated by radiolysis.
generated by radiolysis.
Irisawa, Eriko; Kato, Chiaki; Kamoshida, Michio*; Hakamatsuka, Yasuyuki*; Ueno, Fumiyoshi; Yamamoto, Masahiro
Proceedings of European Corrosion Congress 2017 (EUROCORR 2017) and 20th ICC & Process Safety Congress 2017 (USB Flash Drive), 9 Pages, 2017/09
 -radiolysis of nitric acid solution
-radiolysis of nitric acid solutionIshijima, Yasuhiro; Ueno, Fumiyoshi; Abe, Hitoshi
Nihon Genshiryoku Gakkai Wabun Rombunshi, 16(2), p.100 - 106, 2017/05
Zirconium (Zr) has been used as a structural material at the spent nuclear fuel reprocessing plant in Japan because of its excellent corrosion resistance against nitric acid solution. And the radiolytic hydrogen is known to be generated in the spent nuclear fuel solution. Zr is known to be highly susceptible to hydrogen embrittlement. Therefore, evaluating the radiolytic hydrogen absorption behavior of Zr in nitric acid solution (HNO ) is essential. In this study, immersion tests were conducted on Zr in nitric acid solutions under
) is essential. In this study, immersion tests were conducted on Zr in nitric acid solutions under  -ray irradiation to evaluate its radiolytic hydrogen absorption behavior. Results showed that hydrogen concentration on Zr increased both in 1-3 mol/L HNO
-ray irradiation to evaluate its radiolytic hydrogen absorption behavior. Results showed that hydrogen concentration on Zr increased both in 1-3 mol/L HNO and pure water at 5 and 7 kGy/h after immersion. The amount of hydrogen absorption on Zr under
and pure water at 5 and 7 kGy/h after immersion. The amount of hydrogen absorption on Zr under  -ray irradiation had a direct correlation with the radiolytic hydrogen generation value in HNO
-ray irradiation had a direct correlation with the radiolytic hydrogen generation value in HNO . The results of glow discharge optical emission spectrometry, thermal desorption spectroscopy, and X-ray diffraction result shows that the absorbed radiolytic hydrogen generated a hydride on the surface of Zr.
. The results of glow discharge optical emission spectrometry, thermal desorption spectroscopy, and X-ray diffraction result shows that the absorbed radiolytic hydrogen generated a hydride on the surface of Zr.
Matsueda, Makoto; Irisawa, Eriko; Kato, Chiaki; Matsui, Hiroki
Proceedings of 54th Annual Meeting of Hot Laboratories and Remote Handling (HOTLAB 2017) (Internet), 4 Pages, 2017/00
In the PUREX method, spent fuels are dissolved with nitric acid media. The reprocessing solution containing Fission Products derived from spent fuels is very corrosive to metal materials, the corrosion problem often appears on the surface stainless steel devices. The oxidizing metal ions such as Ruthenium (Ru) and Neptunium (Np) in the process solution is the key reason for severe corrosion of stainless steel. In order to obtain the corrosion rate of stainless steel, we installed the corrosion test apparatus inside an airtight concrete cell in a hot laboratory (the WAste Safety TEsting Facility (WASTEF) of the Japan Atomic Energy Agency), and performed the corrosion tests of stainless steel in the heated nitric acid solution containing Np. The corrosion tests were performed in the temperature range from room temperature to boiling point for 500 hours per batch. The results show that the presence of Np accelerate the stainless steel corrosion in the nitric acid solution.
Kato, Chiaki; Ishijima, Yasuhiro; Ueno, Fumiyoshi; Yamamoto, Masahiro
Journal of Nuclear Science and Technology, 53(9), p.1371 - 1379, 2016/09
Times Cited Count:4 Percentile:36.53(Nuclear Science & Technology)The effects of crystal textures and the potentials in the anodic oxidation of zirconium in a boiling nitric acid solution were investigated to study the stress corrosion cracking of zirconium in nitric acid solutions. The growth of the zirconium oxide film dramatically changed depending on the applied potential at a closed depassivation potential (1.47 V vs. SSE). At 1.5 V, the zirconium oxide film rapidly grows, and its growth exhibits cyclic oxidation kinetics in accordance with a nearly cubic rate law. The zirconium oxide film grows according to the quantity of electric charge, and the growth rate does not depend on the crystal texture in the pretransition region before the cyclic oxidation kinetics. However, the growth and cracking under the thick oxide film depend on the crystal texture in the transition region. On the normal direction side, the oxide film thickness decreases on average since some areas of the thick oxide film are separated from the specimen surface owing to the cracks in the thick oxide. On the rolling direction side, cracks are found under the thick oxide film, which deeply propagate along the RD without an external stress. The cracks under the thick oxide film propagate to the center of the oxide layer. The cracks in the oxide layer propagate in the (0002)Zr plane in the zirconium matrix. The oxide layer consists of string-like zirconium oxide and zirconium hydride. The string-like zirconium oxide contains orthorhombic ZrO in addition to monoclinic ZrO
in addition to monoclinic ZrO . As one assumption for the mechanism of crack initiation and propagation without an external stress, it is considered that the oxidizing zirconium hydrides precipitate in the (0002)Zr and then the phase transformation from orthorhombic ZrO
. As one assumption for the mechanism of crack initiation and propagation without an external stress, it is considered that the oxidizing zirconium hydrides precipitate in the (0002)Zr and then the phase transformation from orthorhombic ZrO to monoclinic ZrO
to monoclinic ZrO in the oxide layer causes the crack propagation in the (0002) plane.
in the oxide layer causes the crack propagation in the (0002) plane.
Irisawa, Eriko; Seki, Masaharu*; Ueno, Fumiyoshi; Kato, Chiaki; Motooka, Takafumi; Abe, Hitoshi
Proceedings of 21st International Conference & Exhibition; Nuclear Fuel Cycle for a Low-Carbon Future (GLOBAL 2015) (USB Flash Drive), p.1108 - 1112, 2015/09
Irisawa, Eriko; Ueno, Fumiyoshi; Uchida, Naoki; Taguchi, Katsuya
Proceedings of 23rd International Conference on Nuclear Engineering (ICONE-23) (DVD-ROM), 3 Pages, 2015/05
Hoshi, Harutaka*; Wei, Y.*; Kumagai, Mikio*; Asakura, Toshihide; Morita, Yasuji
Journal of Radioanalytical and Nuclear Chemistry, 262(3), p.601 - 605, 2005/01
Electroreduction of Tc(VII) in nitric acid solution using grassy carbon electrode was studied. The electroreduction was conducted at a constant potential -300 mV (vs. Ag/AgCl) with a potentiostat. It was found that difference of the Tc concentration in the solutions before and after the electrolysis was negligibly small. This means that there were almost no TcO or Tc deposited on the carbon fiber electrode during the electroreduction. Absorption spectra and distribution coefficients obtained by ion-exchange analysis indicated that Tc(VII) was reduced to Tc(IV).
or Tc deposited on the carbon fiber electrode during the electroreduction. Absorption spectra and distribution coefficients obtained by ion-exchange analysis indicated that Tc(VII) was reduced to Tc(IV).
 -tetraoctyl-3-oxapentanediamide (TODGA) from nitric acid into
-tetraoctyl-3-oxapentanediamide (TODGA) from nitric acid into  -dodecane
-dodecaneZhu, Z.-X.; Sasaki, Yuji; Suzuki, Hideya*; Suzuki, Shinichi; Kimura, Takaumi
Analytica Chimica Acta, 527(2), p.163 - 168, 2004/12
Times Cited Count:263 Percentile:99.08(Chemistry, Analytical)The extraction of 75 elements by  -tetraoctyl-3-oxapentanediamide (TODGA) from nitric acid to
-tetraoctyl-3-oxapentanediamide (TODGA) from nitric acid to  -dodecane was investigated and discussed concerning distribution ratios (D) of metal ions and their ionic radii. The extractability for elements depends strongly on their oxidation state. Monovalent and pentavalent ions were not extractable. In divalent metal extraction, Ca(II) (ionic radius: 100 pm) showed the highest D value and the D decreased with increase or decrease of the ionic radius. Tri- and tetravalent ions with ionic radii of 87-113 pm and 83-94 pm, respectively, showed the D over 1000, and the extracted chemical forms were determined to be M(TODGA)
-dodecane was investigated and discussed concerning distribution ratios (D) of metal ions and their ionic radii. The extractability for elements depends strongly on their oxidation state. Monovalent and pentavalent ions were not extractable. In divalent metal extraction, Ca(II) (ionic radius: 100 pm) showed the highest D value and the D decreased with increase or decrease of the ionic radius. Tri- and tetravalent ions with ionic radii of 87-113 pm and 83-94 pm, respectively, showed the D over 1000, and the extracted chemical forms were determined to be M(TODGA) (NO
(NO )
)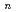 or M(TODGA)
or M(TODGA) (NO
(NO )
)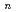 (
( =3 or 4). The oxonium ions with higher oxidation states than tetravalent one forms the complicated metal-complex with TODGA, and the number of TODGA associated in those extraction reactions was confirmed to be 2 at most.
=3 or 4). The oxonium ions with higher oxidation states than tetravalent one forms the complicated metal-complex with TODGA, and the number of TODGA associated in those extraction reactions was confirmed to be 2 at most.
Kida, Takashi; Sugikawa, Susumu
JAERI-Tech 2004-019, 30 Pages, 2004/03
It is known that hydrazine nitrate used in nuclear fuel reprocessing plants is an unstable substance thermochemically like hydroxylamine nitrate. In order to take the basic data regarding the reaction of hydrazine nitrate with nitric acid, initiation temperatures and heats of this reaction, effect of impurity on initiation temperature and self-accelerating reaction when it holds at constant temperature for a long time were measured by the pressure vessel type reaction calorimeter etc. In this paper, the experimental data and evaluation of the safe handling of hydrazine nitrate in nuclear fuel reprocessing plants are described.
Mineo, Hideaki; Isogai, Hikaru; Morita, Yasuji; Uchiyama, Gunzo*
Journal of Nuclear Science and Technology, 41(2), p.126 - 134, 2004/02
Times Cited Count:7 Percentile:45.11(Nuclear Science & Technology)A simple equation was proposed for the dissolution rate of spent LWR fuel, of which the change in the dissolution area was estimated by taking into account of the area of the cracks occurring due to thermal shrinkage of the pellets during irradiation. The applicability of proposed equation was examined using LWR fuel dissolution test results in the present study as well as the results obtained by other workers. The equation showed good agreements with the dissolution test results obtained from spent fuel pellets and pulverized spent fuel. It was indicated that the proposed equation was simple and would be useful for the prediction of dissolution of spent LWR fuels. However, the initial effective dissolution area, the parameter of the equation, was found to depend on the temperature, which could not be explained by the proposed equation. Further studies on the role of other factors affecting dissolution rate, such as nitrous acid, in the dissolution of spent fuel was required.
 -ray
-rayOkagawa, Seigo; Nagai, Hitoshi*; Abe, Hitoshi*; Tashiro, Shinsuke
JAERI-Tech 2003-068, 17 Pages, 2003/08
To study the release mechanism of iodine from the dissolver solution in the nuclear fuel reprocessing plants to the atmosphere at criticality accidents, the characteristics of redox of iodine species in various solutions must be examined. In the present work, the effect of  -ray irradiation on the redox reaction was examined in the nitric acid solution of 1M and 3M. Without irradiation, most of iodine in 1M nitric acid solutions were exist in the form of I
-ray irradiation on the redox reaction was examined in the nitric acid solution of 1M and 3M. Without irradiation, most of iodine in 1M nitric acid solutions were exist in the form of I
 . In 3M nitric acid solutions, iodine was oxidized to I
. In 3M nitric acid solutions, iodine was oxidized to I . When the solutions were irradiated by
. When the solutions were irradiated by  -ray with exposure of more than 4C/kg, I
-ray with exposure of more than 4C/kg, I
 was disappeared regardless of nitric acid concentration. At exposure of 120C/kg, iodine was oxidized to IO
was disappeared regardless of nitric acid concentration. At exposure of 120C/kg, iodine was oxidized to IO
 . At exposure of 4800C/kg, iodine was exist in the form of IO
. At exposure of 4800C/kg, iodine was exist in the form of IO
 in 1M nitric acid solution. On the other hand, iodine in 3M nitric acid solution was reduced to I
in 1M nitric acid solution. On the other hand, iodine in 3M nitric acid solution was reduced to I at the same exposure. In the irradiated solution, nitrous acid was found, which would be produced from nitric acid by
at the same exposure. In the irradiated solution, nitrous acid was found, which would be produced from nitric acid by  -ray irradiation.
-ray irradiation.
Kato, Chiaki
JAERI-Research 2003-013, 143 Pages, 2003/08
This study is investigation about stress corrosion cracking (SCC) of zirconium in nuclear fuel reprocessing. Chapter 1 is described background. Chapter 2 is explained experimental apparates. Chapter 3 is described the increased oxidization potential on the heat-transfer surface and suggested the initiation of SCC on a boiling heat-transfer surface. Chapter 4 is described that the SCC susceptibility increased with increasing nitric acid concentration and solution temperature on notched specimen by SSRT. In addition, the SCC susceptibility effected by the crystal anisotropy by the hot rolling direction and increased on a parallel face to the rolling direction. Chapter 5 is described that the SCC susceptibility increased in HAZ/base metal boundary in order to the preferential orientation of cleavage plane (0002). Chapter 6 is described that the increased oxidization potential on the heat-transfer surface is attributed to the reduction of nitrous acid concentration by the thermal decomposition on the surface and the removal of the decomposition product from solution by boiling bubbles.
Motooka, Takafumi; Kiuchi, Kiyoshi
Journal of Nuclear Science and Technology, 39(Suppl.3), p.367 - 370, 2002/11
A corrosion of a stainless steel in nitric acid solution containing Np has been studied. Using SUS304L stainless steel, corrosion tests in boiling neptunium nitrate solution were conducted under immersion and heat-transfer condition. By the weight loss measurement of stainless steel and the quantitative analysis of metallic ions dissolved in solution, the corrosion rates were obtained. The surface morphology was observed by scanning electron microscopy. The acceleration mechanism was investigated by electrochemistry and spectrophotometry. The corrosion rate was accelerated by addition of neptunium in nitric acid. Preferential intergranular corrosion was observed. The corrosion was enhanced under heat-transfer condition compared to immersion condition. In polarization measurements, the cathodic over-voltage was decreased; the cathodic current was increased by addition of Np. Spectrophotometric measurements showed that Np(V) oxidized to Np(VI) in boiling nitric acid. The corrosion acceleration mechanism in nitric acid solution containing Np suggested the re-oxidation of Np(V).